Advanced Therapies 2025
18-19 March, ExCel, London
Keynote Panel Discussion: State of the Industry Address for CGTs
Matthieu Coutet, Paolo Morgese, Jordan Schecter, MD, Lara Campana, Eric David, Molly Stevens DBE, Miguel Forte
“An event with tradition. Biotech is a vibrant field, but currently like a plane taking off.” – Miguel Forte
Audience questions
Q: Is it easier for cell or gene therapy to get investment?
Autologous therapies have a slight advantage in the funding market as they have already been proven to work and have the data behind them. However, it is a very narrow lead. The current prediction is that in vivo technologies will continue to advance and eventually overtake. It’s a more innovative space than it has ever been over the last decade, and we need to ensure that we’re getting the therapies we have currently to patients whilst we innovate for the future.
Q: How do we lower the price of CGTs?
There’s work to be done to change the narrative around how we demonstrate value. The cost of new therapies may be more expensive initially, but if that therapy means there’s less risk of long-term side effects, which also require treatment (e.g., dialysis), then overall it is a cheaper product (and better for the patient). There’s also work to be done on the manufacturing side to lower costs. If we can reduce that then we’re starting from a lower price point.
My thoughts:
An interesting and inspiring way to start the event. Although CGTs are currently in a period of “winter” it is still a vibrant field, with plenty of innovation and opportunities for funding. It was great to hear that these sentiments are backed up by comments from the big pharma representatives, and that they are still buying and investing in innovative CGT technology “wherever it occurs”. There’s an agreement that the focus should be on streamlining the process of getting therapies to the patients.

Panel Discussion: Assessing the impact of UK and EU relations on the European Advanced Therapies Market
Pamela Tranter, Anike Te, Sean Russell, Miguel Forte, Dr.Salmaan Dalvi
Brexit had a huge initial impact on the CGT industry, but now 5 years after the UK withdrew from the EU, how has the market adapted and changed? The panellists opened It up to the floor for their questions.
Audience questions:
Q: How do we navigate different regulatory authorities?
Differences in regulatory authorities isn’t new and it’s something biotech companies have been contending with for a long time. We should understand the commonalities between the boards before addressing the differences. The positive outlook is that different authorities will require developers to look at things from varying perspectives.
There’s evidence that regulators are still communicating (just in a less formal manner than before), and widely they are the same with a few differences to address. De-risking multi-nationally from the start will help you in the long run as it avoids creating a valley between grades. Consultants are an option, but they are another layer of costing.
Q: How would streamlining trials help?
From a legislative perspective EU harmonisation worked and reduced the time spent on regulatory review. At the moment there’s still a country-by-country basis, even in EU member states, creating multiple routes to market. It’s something the UK can take advantage of and get to market quicker, but this shouldn’t be done at the detriment of the rest of the EU and to patients.
Q: What about the movement of a skilled workforce?
Grants are still available for moving and working across borders, but they are harder to come by. CGT Catapult is a great resource for finding funding. Workforce development is a global need, and countries should be looking to collaborate to make this easier as it benefits everyone (more movement increases perspectives and knowledge transfer). Apprenticeships are also incredibly important as more technicians will be needed as manufacturing sites are established and automation is implemented. There should also be more thought put into which roles can be done remotely to foster growth where free movement isn’t possible.
Q: More skills- quicker processes?
In theory yes, in practice a more skilled workforce creates a smoother, but not necessarily quicker, process. It’s essential that staff are trained in soft skills too and that teams are motivated/excited about what it is they’re working on. It can’t all be left to academia; industry has a role to play and can be more involved in working with universities to address their needs.
Q: What’s the impact of Brexit on funding?
There was a huge dip in funding applications at the start as people didn’t know what would be available to them anymore. Public funding is more challenging these days across the board, and increasingly so due to political climates. Funding for rare diseases is even more uncommon. In the EU public funding is a more hopeful environment, whereas it’s incredibly challenging in the US. There’s scope for the EU and UK to collaborate and take advantage of this.
My thoughts:
Undoubtedly the advanced therapies market has changed and been challenging over the last few years. But there are promising signs for the future for both the UK and the EU. The UK has the potential to take advantage of its position and get to market quicker, however, without collaboration and ease of patient and workforce movement, this market speed won’t account for much of an advantage.

Start Up Showcase: Journey from Academic Spin Out to the Clinic
It’s tempting to look at the ATMP pathway as a checklist of items you need to do in order. However, in reality there’s a lot of interplay between them all and some key questions you need to consider.
Starting with the end in mind is crucial, and funding is typically stage-gated, with key inflection points to consider. It’s important to implement stage-appropriate processes and collaborate with experts to leverage their knowledge.
Choosing the CDMO is vital, and each provider has their pros and cons. But remember, you know your product best!
Regulatory requirements vary by country, so strategic planning is necessary to avoid inadvertently excluding potential markets. Early engagement with authorities and thorough CMC risk assessments are essential.
When evaluating data, consider signs of efficacy and whether there is room for innovation through identifying gaps in the market. Additionally, assess the commercial viability by comparing reimbursement value to the cost of goods, to ensure that clinicians will prescribe, and patients will use your product.
Panel Discussion: Staying Agile and Embracing Technological Innovations to Optimise Cell Therapy Manufacturing
Jason Beckwith, Maitane Ortiz Virumbrales, Łukasz Kwiatkowski, Katy Newton, Brian Philip
There was some discussion about whether the “product is the product” or if “the process is the product” general consensus being that it’s complicated and when you’re at the start of the journey it can be hard to determine.
When discussing how to deal with redundancies the panel had a variety of strategies including building in the redundancies as a “just in case” scenario to make sure that if you have the capacity to test more than one thing, then do. More data isn’t going to be a bad thing, especially in the long run. Another is to split your resources among supplies in a 70/30 split, then you know that both have gone through the process and created good product, and you have a backup if needed.
Tech innovation is rapid at the moment, with lifespans of 5-10 years and shortening. So what alternatives are there for equipment redundancies? Start validating a new equipment whilst legacy kit is still running to make sure you have an overlap should something go wrong. Modular systems are most popular these days as they are easier to modify and switch out. Single use kits are also an option, but they are less economical and sustainable.
My thoughts:
It was really interesting to hear the thoughts from the panel as to how we can manage such a rapidly changing field, both from a hardware perspective and through process. The discussion surrounding what your product actually is (process or product) was insightful. While newcomers to the CGT sector might find these concepts complex, the proposed solutions, though not all-encompassing, can significantly enhance a company’s agility in this dynamic field when implemented with foresight and strategic planning.

Panel discussion: The affordability of ATMPs: Striking the balance between commercial interests and patient uptake
Sven Kili, Ben Doak, Katja Berg, Catriona Crombie, Joshi Venugopal
Starting off with the big question- are cell and gene therapies (CGTs) worth it in the current political landscape? CGTs are curative therapies so they are worth developing from both a patient and healthcare perspective. However, the industry needs to find a way to improve the commercial feasibility of CGTs and increase patient access.
The industry is going through a Darwinian survival of the fittest in terms of the multitude of CGTs candidates and how commercially viable they are. One of the pitfalls that the industry needs to try and avoid is looking back over the past few decades (when there was lots of investment) as the ‘good old days’ of CGTs. The current scarcity of investment can be seen as an opportunity to be more thoughtful about where the industry focuses on. Eventually there will be a point at which the commercial viability and patient access/uptake will balance out.
Patient access doesn’t just equate to affordability for healthcare systems though. Having a good understanding of the patient community for a therapy is crucial. Although CGTs have amazing potential some patients may not want to try newer therapies, so it’s important to bring patients in at an early stage (like including them in conversations at conferences like this one).
What challenges face the industry and how can we overcome them?
- New ways of working: CGTs often requires adapting ways of working to fit with healthcare systems in the first instance. In the case of inhalable insulin, there was a patient need, but it flopped because the system wasn’t ready for it. For now, the industry should focus on developing therapies for severe diseases that have no treatment options, because the bar for change is quite high. Once the technology has evolved and the infrastructure has been established, therapies for other diseases can be developed.
- Data, data, data: the industry needs to generate and analyse lots of data to demonstrate the long-term efficacy and safety of CGTs. Alongside data collection, methods and infrastructure for data storage, processing, and analysis need to be developed to optimise data handling and gain insights.
- Are the practical? The industry needs to consider the practicalities of the therapies they’re developing and how they will impact patients and HCPs. This could be done through early engagement with the NHS and utilisation of the resources they provide.
- Looking at the whole picture: The entire value chain in the CGT industry needs to be optimised to improve efficiency. Success of the industry will come from marginal gains across the supply chain.
- Stand out and fit in: Just because we in the industry think CGTs are amazing, doesn’t mean everyone in the world does. For the industry to be competitive it needs to fit within the current logistics systems. We’re not quite as special as we think we are.
Audience questions:
Q. We are only as good as our payers to get to our patients. What are the weaker points in that ecosystem and how can stakeholders do more?
There are opportunities to make gains across the value chain, for example more efficient collection and use of data (both in and beyond the healthcare system) and utilising the NHS in a better way. We need to change the mindset globally to convey that these therapies are an investment. The barriers are very different depending on what system you are working in, e.g., private insured healthcare systems, low-middle income systems. For approved gene therapies, one common element that can improve outcomes is diagnostics and infant screening to identify patients earlier. The sector is already highly collaborative, so companies need to take advantage of the opportunities out there.
Q. It’s tough to get products reimbursed. How can we do that better?
In England, NICE assesses products for affordability and they’re very transparent about the processes they use. Developers can, therefore, learn from other products that have already gone through the process. When developing a therapy, you need to think about what you’ll need for reimbursement e.g. real-world evidence, screening for diseases, at the beginning. The UK has a more favourable position in reimbursement than Europe for ATMPs, but there is a lack of infant screening for diseases. Many companies will want to collect feedback and build trust within their products through early access programs, but these are easier to implement in certain geographies (Europe and the US) than others (the rest of the world).
Q. Affordability and patient uptake is linked to the investor community. How can the industry educate the investor community to make affordability a reality?
Developers need to pitch high unmet needs to investors rather than trying to raise funding for all diseases. For investors, finding the maximum value for investment might mean getting involved in specialist areas. The vast majority of investors haven’t run a company, so developers need to break down the science, the product, and the landscape e.g., patient audience, clinician education, route to exit.
Q. How does the curative nature of CGTs fit the business model of a pharmaceutical company?
This question should be reframed to say how can the pharma model fit the curative nature of CGTs? The reality is things change and markets dry up But there will always be conditions to treat. We need to embrace when diseases are cured.
My thoughts:
This was a great discussion with a variety of viewpoints from the panellists. Sven Kili is an experienced leader in the field, and he was able to ask the questions that got straight to the nitty gritty of the challenges facing CGTs from both a commercialisation and patient uptake perspective.

Gene therapies: a futuristic perspective
Where does gene therapy fit? When showing the room 3 adoption curves, most of the room fell on the middle curve as the one that best described the adoption of gene therapies (there was only one person in the room who had a more pessimistic view).
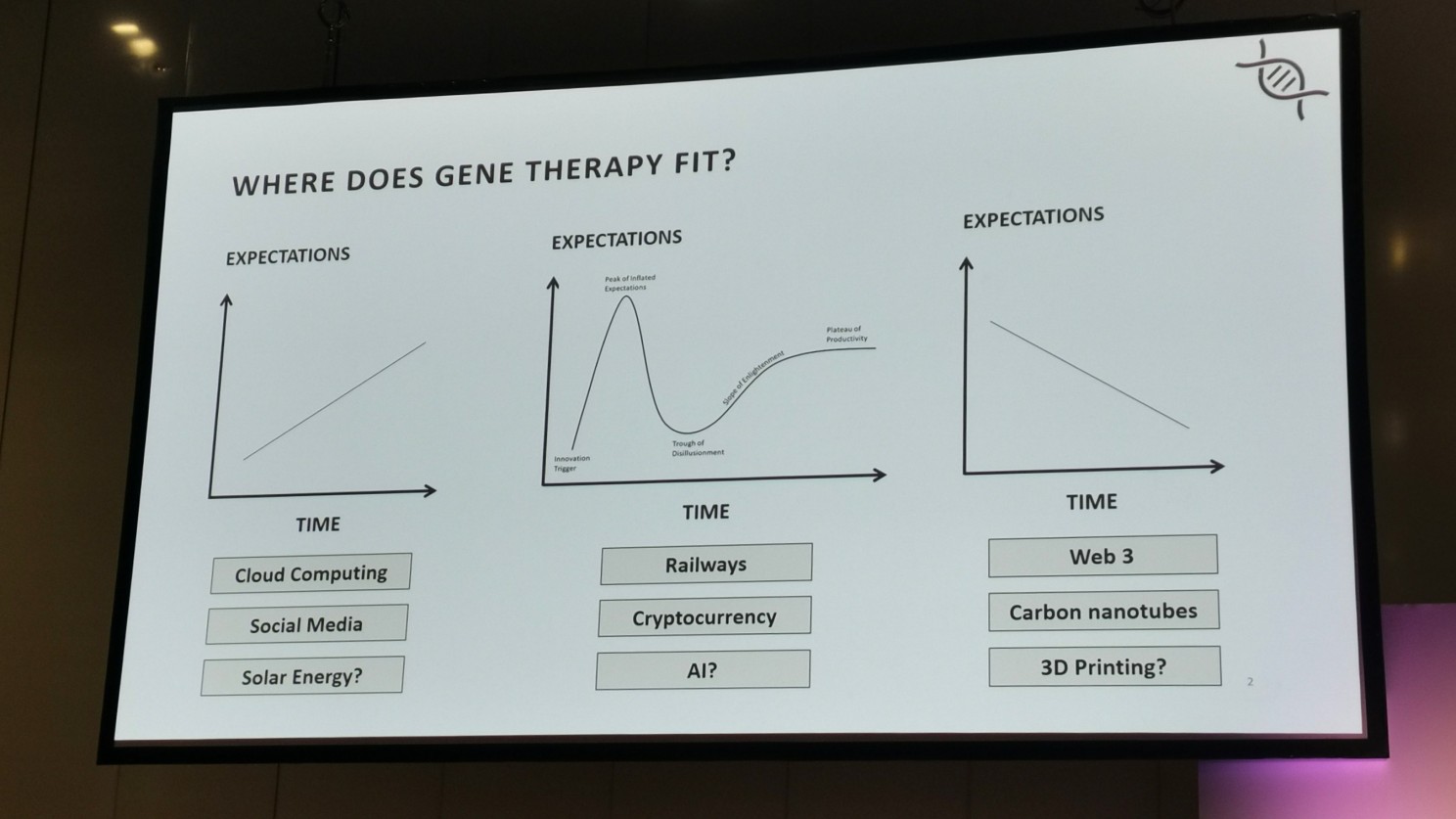
Joshi framed his talk around 3 inflexion points that are shaping the gene therapy landscape.
Inflexion point 1: Whole genome screening
The concept of genome sequencing for new-borns is emerging but is a vital step for the widespread adoption of gene therapies.
The potential benefits of new-born screening include:
- Expanding screening for more conditions/genes
- Fast addition of new conditions/genes
- Reduce national disparities in NBS
- Genetic data for adult care
- Treasure trove of longitudinal data for research
The potential concerns of new-born screening include:
- Data privacy
- Ethical issues (e.g., consent)
- Cost-effectiveness
- Harm caused (e.g., stress)
- Health services demand rise
Inflexion point 2 – Technology
Technology frontiers include non-viral vectors, gene editing, and scalable manufacturing. Aspects of these challenges include safety, tissue trophism, and cost (non-viral vectors); regenerating cells, gain of function, and immune response (gene editing); coast per unit, consistency, and speed (scalable manufacturing).
Viral vectors dominate when it comes to the development gene therapy products with 70% of products involving gene editing and 30% involving gene replacement. The most common therapeutic areas for gene therapies include:
- Neurological disorders (20.1%)
- Blood disorders (17.7%)
- Eye disorders (23.7%)
- Cardiovascular diseases (8%)
- Immune-mediated disease (7.6%)
Inflexion point 3 – Commercialisation
Companies with a European commercial footprint make up over 90% of CGT companies and the European market should be as attractive as the US market in terms of target population for gene therapies. However, revenue share and shareholder returns from gene therapies indicate that this isn’t the case. Many rare disease products also don’t meet the commercial threshold for big pharma – blockbuster drugs are rare but create value, whereas smaller products dilute value.
My thoughts:
Joshi gave a whistlestop tour of the current landscape and future perspectives of gene therapies. It was interesting to see what the rest of the room thought about the adoption of gene therapies (and props to the person who volunteered his more pessimistic view in a room full of optimists and realists).

Harnessing automation, analytics, and scalability to drive innovation
There is a big push on manufacturing automation reflecting the needs of the CGT industry – lower labour costs, less manual intervention to reduce contamination risk, integration into existing manufacturing processes, and allowing easy scale up of manufacturing processes.
To date, there hasn’t been much innovation in process development. Currently there are very limited bioprocess tools for small-scale development, and full-scale tools are way too expensive to buy and run at the process development stage.
Analytics technology exists, but it’s integration within current systems is a challenge. Data is crucial to process development, and although the technology to collect data like pH & dO2, cell counts and viability, and potency is out there, using it on a day-to-day basis is cumbersome.
Practically, the ideal scenario is generating high quantities of data at small scales to refine a process. Then, when it’s time to manufacture, the process can be understood, scaled up using the exact same parameters (measured by integrated analytics), and automated to reduce variables and increase manufacturing efficiency. If there is need for further refinement, the process should be able to be easily scaled down.
The MFX bioreactor is scalable by design via parallelization, enabling it to be readily scaled up and down. It can then be automated to drive manufacturing. They can get thousands of data points from one 8ml bio reactor, and their T-cell expansion data showed that their bioreactors demonstrated a 7-fold improvement in cell growth, was fully automatable, and cost 65x less than a standard manufacturing scale run.
Audience questions:
Q. Have you considered how your bioreactor/platform integrates in a manufacturing process?
The goal for MFX is to develop an end-to-end platform whilst integrating existing technology at certain points. They are trying to avoid cell transfer and other complexities that increases CapX to bring the maximum value to CGT developers.
My thoughts:
MFX are one of Mowbi’s clients, so I was familiar with their technology, but Antoine did a good job of breaking down the needs of developers, what is already out there, and what MFX are trying to do with their platform.

Panel discussion: Best practice for patient centred clinical trial design
Sven Kili, Jaap Jan Boelens, Trish Parry, Su Xiao, Renaud Valliant
Jumping in with a big question – are the EU/US trial frameworks fit for purpose? The panel generally agreed that the current frameworks are fit for purpose, but the optical landscape is changing, which is affecting clinical trials. Companies also want to get to clinical trials quickly, so they may be looking further afield to start collecting data.
Places like Australia are attractive to begin phase 1 trials as they have plenty of incentives (e.g., tax breaks) and retain good quality so the collected data will be acceptable to continue on with. Whilst this is good news for companies, the EU and US might want to take note of this and work on making the landscape more attractive for early-stage companies to start clinical trials. For ultra-rare diseases it’s hard to recruit patients in the US and EU, so places like China where it is easier to recruit patients might be a more attractive option.
Q. How easy is it to bring an outside clinical trial back into the FDA or EMA?
For Australia, because it’s part of the ICH, it’s easily accepted by regulatory bodies. For countries outside the ICH it’s harder to align the data and for IITs it’s a mixed bag. There’s a move to use more RWE to support applications, but it all depends how you capture the data. Because of the practices in China, the data collected is usually used as supportive, not primary data.
When looking at patient-centric studies, it’s essential to ask patients for outcomes, even if they are only able to be incorporated into secondary endpoints. But primary endpoints still need to be scientifically achievable and statistically significant. For rare diseases, placebo arms should be built into phase 2 trials to mitigate potential placebo effects before the pivotal trials begin. More flexibility and the adoption of new technologies is important to allow successful enrolment and retention of patients in clinical trials.
Is the approach by regulators to start accepting biomarkers as key outcomes and indicators of effect a good approach? Biomarkers can certainly be very informative and in rare diseases where endpoints can be 5 years away, they’re going to be helpful and move the field forward faster. They can also be useful as data for investors to keep biotechs afloat while they work towards their primary endpoints. But the important thing is that biomarkers need to be validated, which will be an additional burden on biotechs.
My thoughts:
The expertise of this panel made for a lively and informative discussion around patient centred clinical trial design. Although Australia and China are becoming more attractive for early-stage companies to start their clinical trials, the time difference is going to hit hard! It was interesting to hear the optimism from some of the panellists around biomarkers balanced out with the realism of others. And of course, what starts a panel session better than a slow clap for one of the panellists who turns up a bit late?

Final thoughts
Advanced Therapies was a bonanza of an event, but their conference app allowed you to easily set your personalised agenda over the 2 days, which was very helpful. The venue was huge, and it packed in a lot of presentation areas, designated meeting points, exhibitors, and a rest and recharge area. It was also the first time I’d used silent seminar (I think that’s what they’re called), and it took me a minute to figure out why people were wearing headphones! Advanced therapies is well established as a key event in the field, so it was inevitable to bump into some old friends and connections, and meet new innovators in the field. If you’re getting into the advanced therapies field, mark this event as one to go to!
